Have you observed the following? Let’s say you’re using TCM199, or another 25 mM bicarbonate buffered medium containing phenol red as the pH indicator. As soon as you remove the medium from the 5-6% CO2 incubator, you begin to see whisps of magenta-colouring within the medium. In a matter of minutes, the medium has changed from the desired “salmon-pink” colour to a purple colour, indicating that your medium has shifted from pH 7.2-7.3 to above pH 8! Why does it increase so quickly? Removing the source of CO2 interrupts the equilibrium established between free hydrogen ions (H+, or protons, the determinant of pH) and bicarbonate ions (HCO3–) with dissolved carbonic acid (H2CO3), causing alkalinity of the medium as the level of carbonic acid is depleted. The reaction can be summarised as follows:
CO2(gas) + H2O(liq) ↔ H2CO3(aq) ↔ HCO3–(aq) + H+(aq)
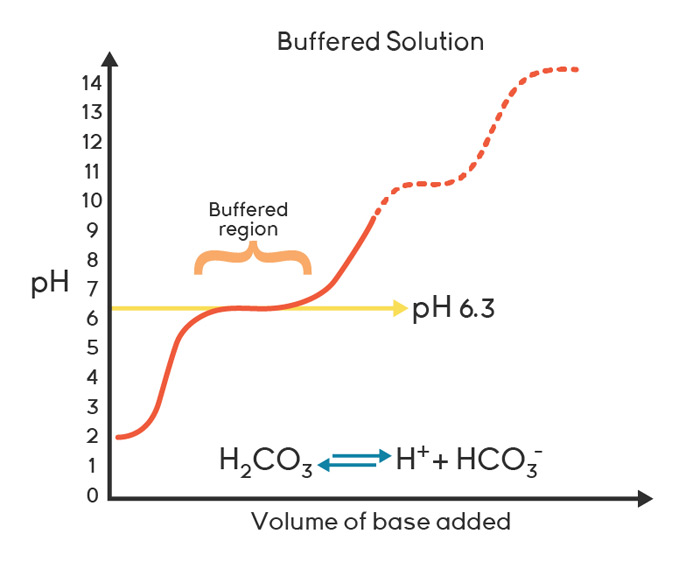
Image sourced from Chegg
Because of this reaction, the carbonic acid equilibration has pH buffering capacity, whereby addition of other acids or alkalines, dependent on the concentration of CO2 and HCO3–, causes the reaction to move to the left or right, assisting in preventing fluctuations of pH. This system is the “natural” blood and interstitial fluid buffer. There are other buffer reactions, such as associated with phosphate and glycine, but none are as important for cells as this system is linked to respiration. But all buffers have a pH “sweet spot”, where they are more effective at buffering against a pH change in the face of mounting acid or alkaline conditions. This sweet spot is termed the pKa. The pKa describes the dissociation constant for protons from a soluble acid-base molecule. Carbonic acid has two pKa’s (as it has 2 available protons); one at pH 6.3 and another at 10.3.
So, what does this mean for our culture systems? Firstly, if equilibrated at pH 7.2 with bicarbonate/CO2, then the pH change in the face of acidification will be less than that of alkalinity. Secondly, combining other buffers into the culture medium that have pKa’s around 7-7.4, even in addition to bicarbonate/CO2 buffering, will further stabilize the pH shift in the face of acidification or alkalinity. Examples are the widely used zwitterionic buffers, HEPES and MOPS.
Our media always contains an amount of MOPS buffer, the concentration of which depends on whether it is for use in air or in combination with bicarbonate/CO2 buffering, for exactly these reasons.
