Our breakthrough in IVP technology
With our media, over 70% of fertilized oocytes form good quality blastocysts. Industry standard rates rarely exceed 40%.
Our Breakthrough
What is your lab’s current cattle IVP blastocyst rate from abattoir-collected oocytes? 30% from fertilized oocytes? Or is it more like 40%, or even 50%? What about over 70%? Have you ever seen those results over several runs, months apart?
We have made a breakthrough in IVP technology, where over 70% of fertilized oocytes (over 60% of all oocytes) form good quality blastocysts. And no CO2 atmosphere or special gas mix is required for IVM, making it ideal for “Transport IVM”.
The high development rate (>70%) from our media is a result of over 30 years of research, development and industry consultation, led by the company’s founder, Professor Jeremy Thompson.
Industry standard rates rarely exceed 40%.
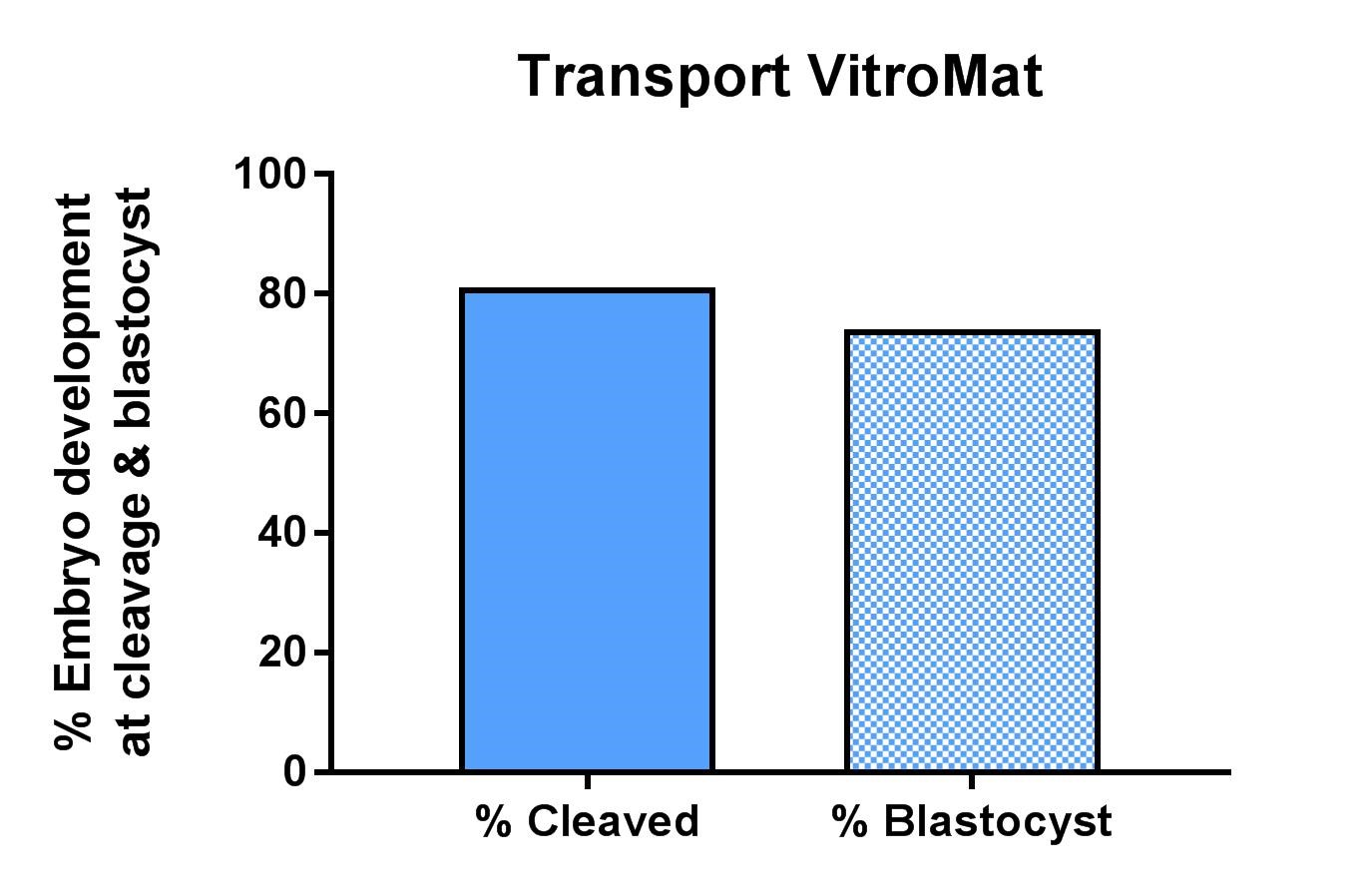
COCs matured in Transport VitroMat for 24 hours in 38.5°C environment. Represented as pooled data from 230 COCs over several experimental replicates.
Bovine Embryo Development Results
The high development rate >47% blastocyst development from oocyte number, >60% blastocyst development from cleaved number of embryos is a result of over 30 years of research, development and industry consultation led by the company’s founder Professor Jeremy Thompson.
Industry standard rates rarely exceed 40% blastocyst development from cleaved embryos.
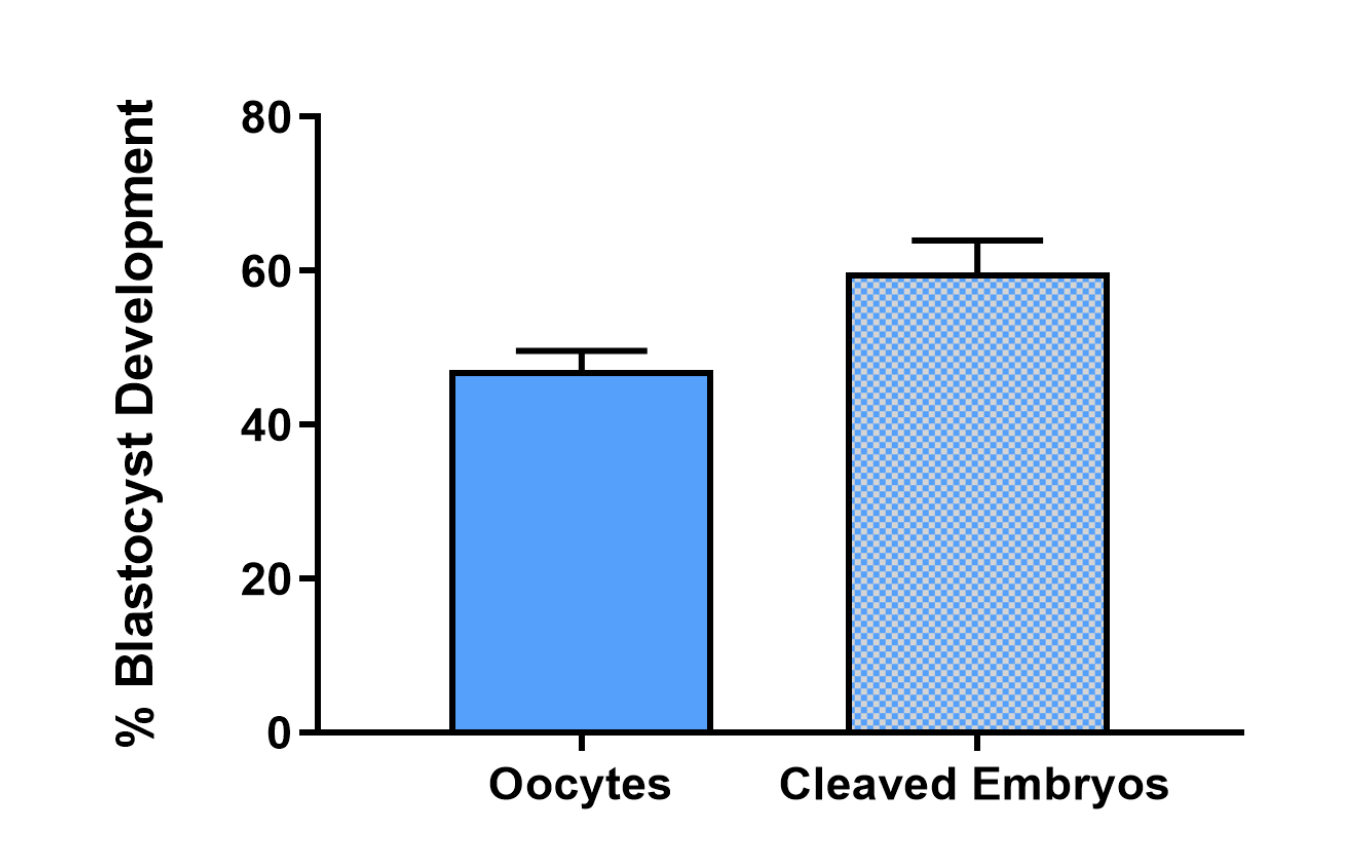
Graph represents percent blastocyst development from oocytes and cleaved embryos via bovine embryo assays performed externally by an independent laboratory in Australia. Representative of 909 COC’s over several replicates. COC’s were aspirated from abattoir-derived ovaries and cultured in a serum free system using ART Lab Solutions complete bovine media suite. Zygotes were then cultured in vitro until day 8 of embryo development, cleavage and blastocyst rates were recorded.
Greater blastocyst development
Our base IVM media resulted in 10% greater blastocyst development over published commercially available media.
The below experiment compares the ART Lab Solutions VitroMat maturation medium and a well known commercially available maturation medium on bovine embryo developmental competence. This research was conducted by an experienced bovine embryologist at the University of Adelaide, South Australia.
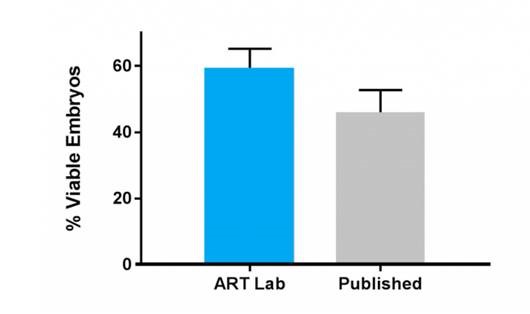
ART Lab Solutions base IVM media resulted in 10% greater blastocyst development over published commercially available media. Data presented as mean ± SEM, n= 14 individual experiments, representative of 567-577 cumulus-oocyte complexes per treatment group. Embryos were assessed and results were recorded on embryonic day 8 post insemination, blastocysts were graded accordingly. Both groups used VitroFert, VitroWash, VitroCleave and VitroBlast for the remainder of the culture period. All media were supplemented with 4mg/ml bovine serum albumin, no further serum was used (fetal calf/bovine serum) .
Quality Control & Results
We apply the highest standards to all stages of media manufacture and quality assurance.
Our products are quality control tested via assessment of Osmolality (mOsm/kg), pH at 38.5°C, and performance of a Bovine Embryo Assay and a Mouse Embryo Assay.

Bovine Embryo Assay
All media manufactured by us is quality control tested using the Bovine Embryo Assay (BEA) to ensure quality and consistency of media production. This assay is performed by an independent laboratory in Victoria using abattoir-derived ovaries. In vitro culture conditions are serum-free and embryos are cultured for 8 days.
To obtain a copy of our Certificate of Analysis for any batch of media please contact us directly.
Mouse Embryo Assay
Our media is quality control tested using the Mouse Embryo Assay (MEA). Embryos are cultured in sample media in the presence of protein from day 1-2, and in the absence of protein from day 2-5 of embryo culture. Positive and negative control results are provided with the Certificate of Analysis to further validate testing.
To obtain a copy of our Certificate of Analysis for any batch of media please contact us directly.
Papers using our founder Professor Jeremy Thompson’s formulated cattle in vitro embryo production media
We have a repository of Cattle IVP Embryo papers featuring our Founder Jeremy Thompson, supporting ART LAB Solutions media development.
- Monteiro, C.A.S., Chow, D.J.X., Leal, G.R, Tan, T.CY.,Ferreira, A.M.R., Thompson, J.G (2021) Optical imaging of cleavage stage bovine embryos using hyperspectral and confocal approaches reveals metabolic differences between on-time and fast-developing embryos. Theriogenology. 159:60-68.
- Whitty, A., Kind, K.L., Dunning, K.R., Thompson, J.G. (2021) Effect of oxygen and glucose availability during in vitro maturation of bovine oocytes on development and gene expression. Assisted Reproduction and Genetics. 38: 1349-1362.
- Soto-Heras, S., Paramio, M.T., Thompson, J.G (2019) Effect of pre-maturation with C-type natriuretic peptide and 3-isobutyl-1-methylxanthine on cumulus-oocyte communication and oocyte developmental competence in cattle. Animal Reproduction Science. 202:49-57.
- Sutton-McDowall, M.L., Gosnell, M., Anwer, A.G., White, M., Purdey, M., Abell, A.D., Goldys, E.M. and Thompson, J.G. (2017) Hyperspectral microscopy can detect metabolic heterogeneity within bovine post-compaction embryos incubated under two oxygen concentrations (7% versus 20%). Human Reproduction. 32:2016–2025.
- Li, H.J., Sutton-McDowall, M.L., Wang, X., Sugimura, S., Thompson, J.G.and Gilchrist, R.B. (2016) Extending prematuration with cAMP modulators enhances the cumulus contribution to oocyte antioxidant defence and oocyte quality via gap junctions. Human Reproduction 31:810-821.
- Sutton-McDowall, M.L., Lu, L.L.Y., Purdey, M., Abell, A.D., Goldys, E. MacMillan, K.L., Thompson, J.G. and Robker, R.L. (2016) Nonesterified fatty acid-induced endoplasmic reticulum stress in cattle cumulus oocyte complexes alters cell metabolism and developmental competence. Biology of Reproduction 94(1):23. doi: 10.1095/biolreprod.115.131862.
- Sutton-McDowall, M.L., Purdey, M., Brown, H.M., Abell, A.D., Mottershead, D.G., Cetica, P.D., Dalvit, G.C., Goldys, E.M., Gilchrist, R.B., Gardner, D.K. and Thompson, J.G. (2015) Redox and anti-oxidant state within cattle oocytes following in vitro maturation with bone morphogenetic protein 15 and follicle stimulating hormone. Molecular Reproduction and Development. 82:281-94.
- Sugimura, S., Ritter L.J., Rose R.D. and Thompson J.G., Smitz J., Mottershead D.G., Gilchrist R.B. (2015) Promotion of EGF receptor signaling improves the quality of low developmental competence oocytes. Developmental Biology. 403:139-49.
- Machado, M.F., Caixeta, E.S., Sudiman, J., Gilchrist, R.B., Thompson, J.G., Lima, P.F., Price, C.A. and Buratini, J. (2015) Fibroblast growth factor 17 and bone morphogenetic protein 15 enhance cumulus expansion and improve quality of in vitro-produced embryos in cattle. Theriogenology 84:390-398.
- Green, M.P., Harvey, A.J., Spate, L.D., Kimura, K., Thompson, J.G. and Roberts, R.M. (2015) The effects of 2,4-dinitrophenol and D-glucose concentration on the development, sex ratio, and interferon-tau (IFNT) production of bovine blastocysts. Molecular Reproduction and Development 83: 50-60.
- Thompson, J.G., Gilchrist, R.B., McDowall, M.L. (2014) Metabolism of the bovine cumulus oocyte complex revisited. In: “Reproduction in Domestic Ruminants VIII”L. Juengel, A. Miyamoto, C. Price, L.P. Reynolds, M.F. Smith, R. Webb, pp 311-326. Context Products, Leicestershire, UK.
- Sutton-McDowall, M. L., Yelland, R., Macmillan, K. L., Robker, R. L. and Thompson, J. G. (2014) A study relating the composition of follicular fluid and blood plasma from individual Holstein dairy cows to the in vitro developmental competence of pooled abattoir-derived oocytes. Theriogenology 82:95-103.
- Sudiman, J., Sutton-McDowall, M.L., Ritter, L.J., White, M.A., Mottershead, D.G., Thompson, J.G., Gilchrist, R.B. (2014) Bone morphogenetic protein 15 in the pro-mature complex form enhances bovine oocyte developmental competence. PLoS One. 2014 Jul 24; 9(7):e103563.
- Sugimura, S., Ritter, L. J., Sutton-McDowall, M. L., Mottershead, D. G., Thompson, J. G. and Gilchrist, R. B. (2014) Amphiregulin co-operates with bone morphogenetic protein 15 to increase bovine oocyte developmental competence: effects on gap junction-mediated metabolite supply. Molecular Human Reproduction. 20:499-513.
- Morado, S., Cetica, P., Beconi, M., Thompson, J. G., Dalvit, G (2013). Reactive oxygen species production and redox state in parthenogenetic and sperm-mediated bovine oocyte activation. Reproduction 145: 471-478.
- Caixeta, E. S., Sutton-McDowall, M. L., Gilchrist, R. B., Thompson, J. G., Price, C. A., Machado, M. F., Lima, P. F., Buratini, J. (2013). Bone morphogenetic protein 15 and fibroblast growth factor 10 enhance cumulus expansion, glucose uptake, and expression of genes in the ovulatory cascade during in vitro maturation of bovine cumulus-oocyte complexes. Reproduction 146: 27-35.
- Gutnisky, C., Dalvit, G.C., Thompson, J.G., Cetica, P. (2013) Pentose phosphate pathway activity: Effect on in vitro maturation and oxidative status of bovine oocytes. Reproduction, Fertility and Development. 26: 931-942.
- Gutnisky, C., Morado, S., Dalvit, G. C., Thompson, J. G., Cetica, P. D. (2013). Glycolytic pathway activity: effect on IVM and oxidative metabolism of bovine oocytes. Reproduction, Fertility and Development 25: 1026-1035.
- Sutton-McDowall, M.L., Feil, D., Robker, R.L., Thompson, J.G., Dunning, K.R. (2012) Utilisation of endogenous fatty acid stores for energy production in bovine pre-implantation embryos. Theriogenology 77:1632-41.
- Sutton-McDowall, M.L., Mottershead, D.G., Gardner, D.K., Gilchrist, R.B., ThompsonG. (2012) Metabolic differences in bovine cumulus oocyte complexes matured in vitro in the presence or absence of follicle stimulating hormone and bone morphogenetic protein 15. Biology of Reproduction 87: (87)1-10.
- Albuz F.K., Sasseville M., Lane M., Armstrong D.T., Thompson J.G., Gilchrist R.B. 2010. Simulated physiological oocyte maturation (SPOM): A novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Human Reproduction 25: 2999-3011
- Hussein, T.S., Sutton-McDowall, M.L., Gilchrist, R.B., Thompson, J.G. (2011) Temporal effects of exogenous oocyte-secreted factors on bovine oocyte developmental competence during IVM. Reproduction, Fertility and Development 23: 576-84
- Lopes A.S., Lane M., Thompson J.G. (2010) Oxygen consumption and ROS production are increased at the time of fertilization and cell cleavage in bovine zygotes. Human Reproduction 25: 2762-2773
- Irving-Rodgers, H.F., Morris, S., Collett, R.A., Peura, T.T., Davy, M., Thompson, J.G., Mason, H.D., Rodgers, R.J. (2009) Phenotypes of the ovarian follicular basal lamina predict developmental competence of oocytes. Human Reproduction 24:936-44
Let us help you
We deliver reproductive technologies that accelerate the improvement of livestock quality, and we’d like to help you do just that.
